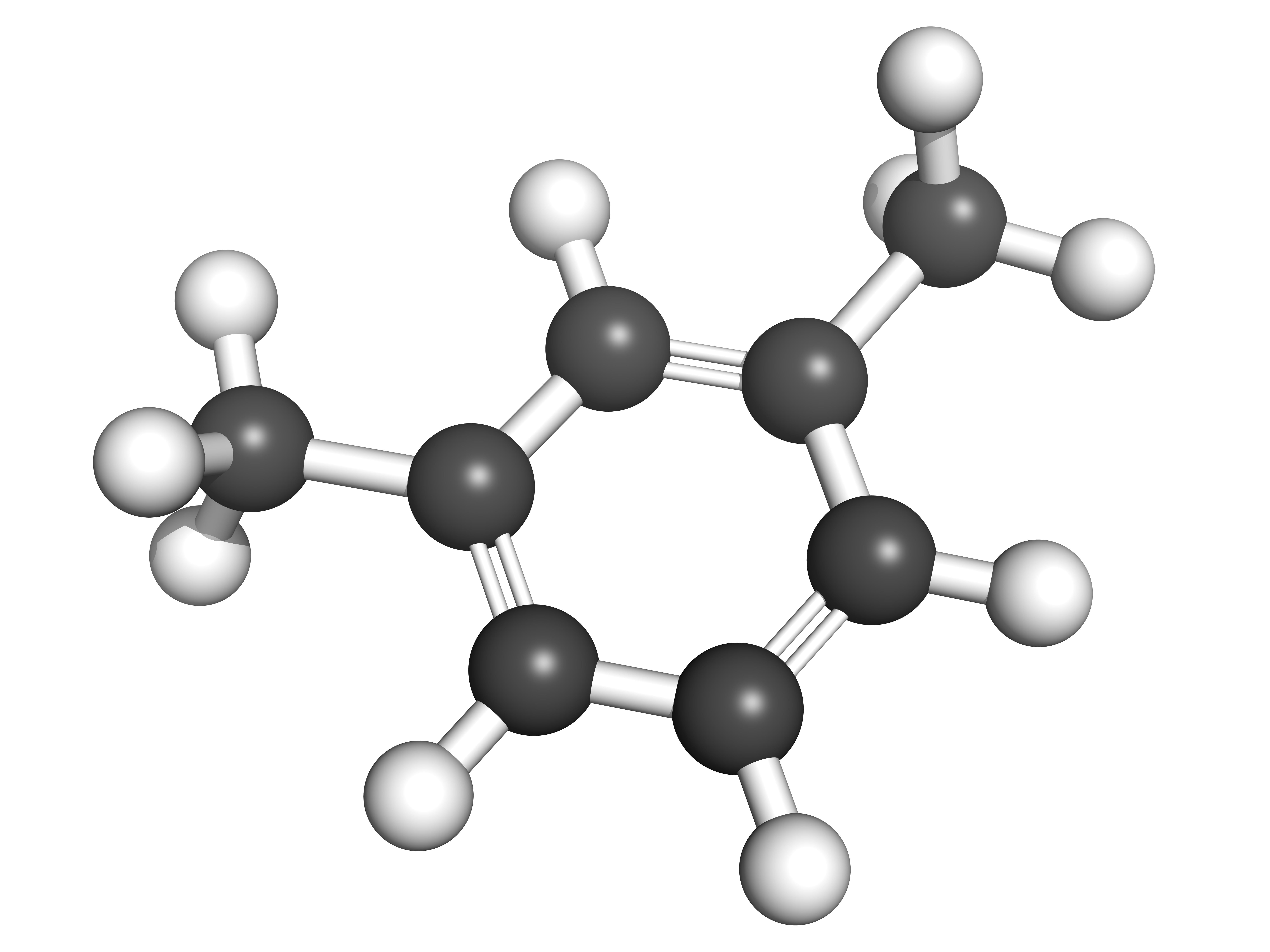
Xylene remained unidentified and innominate until 1850, when a distinguished French scientist made its discovery… His name was Auguste Andre Thomas Cahours, and his contribution to organic chemistry was among the greatest in history…
Auguste Cahours discovered the processes of synthesis in various chemical molecules including toluene, several organo-magnesium’s and in derivatives of phosphine and arsine. Xylene was among these, having been discovered as a constituent of wood tar.
He named this peculiar liquid after “xylong”; the Greek word for “wood”. Over time, it has come to be known as xylene.
What are the qualities of xylene?
Xylene is a colourless, flammable and greasy liquid with a treacly odour. It also gives off its gas and is heavier than air.
Xylene exists in three forms: metaxylene, orthoxylene and paraxylene. These are known as isomers.
Is it hazardous?
The nauseously sweet smell of xylene is a sign of its toxic nature. For the general population, low level exposure to xylene occurs from cigarette smoke and vehicle exhaust emissions. However, individuals may also breathe in small amounts of xylene when using certain cleaning products. Severe exposure to xylene should be avoided at all costs because symptoms can be deadly.
What are the symptoms?
Moderate exposure to xylene can cause mild irritation to the skin, eyes, nose, and throat. It can further trigger headaches, dizziness, confusion, and loss of muscle coordination.
Severe exposure can generate sleepiness, stumbling, irregular heartbeat, fainting or in extreme cases, death.
Who is most at risk?
Those who are at most risk to xylene work in the following industries:
- Painting and furniture refurbishment (working with paint thinners, solvents, and paint removers)
- Biomedical laboratory workers who use it as a solvent to fix tissue specimens and rinse stains
- Xylene distillation and purification
- Using xylene as a raw material
- Gas station and garage workers through frequent exposure to xylene
Where would I find it?
Xylene occurs naturally in petroleum products and crude oils. It is further used as an industrial solvent in printing, rubber, and leather industries. Industrial demand for xylene is rather high because of consumer demand for plastic products. Recent research has shown that global demand for xylene will rise to about 63.6 million tonnes by 2023.
It is also used as a cleaning agent for steel and electronic components like silicon wafers and integrated circuits.
It is rather common for foods to contain trace amounts of hydrocarbons, including xylene. These include meat, poultry, eggs, milk, bread, green vegetables, potatoes, and nuts.
How can I protect myself?
In the workplace, limit exposure by enclosing operations and ensure that all spaces are well-ventilated. If adequate ventilation is not possible, wear a respirator.
When handling xylene, correct Personal Protective Equipment (PPE) is required. This includes chemical safety goggles and protective clothing (gloves, aprons, boots), facemasks or face shields.
If you or someone you know is concerned that they may be at risk from exposure to xylene, please use the form below to get in touch with Healthscreen UK today. Alternatively, call 014555245743 to get in touch with one of our dedicated specialists.